Abstract
Introduction: Follicular lymphoma (FL) comprises a heterogeneous group of diseases. Typical FL (TFL) most commonly presents with advanced stage (AS), has a variable clinical course, and is incurable. A small subset of TFL cases present with limited stage (LS) disease, and may be cured with radiotherapy. Primary cutaneous follicular lymphoma and pediatric-type follicular lymphoma (PTFL) are non-typical FL subtypes with localized presentation and invariably benign behavior. In contrast to ASTFL, these subtypes lack BCL2 -rearrangements. We recently discovered that, in contrast to typical FLs, the majority of PTFL cases are driven by mutations activating the MAP kinase pathway and lack mutations in epigenetic modifier genes (Louissaint, Blood 2016; Schmidt, Blood 2017).
Primary intestinal FL (PIFL) is another non-typical variant of FL, characterized by its distinctive restriction to the intestine (most commonly the duodenum). Despite this striking behavior, PIFL is indistinguishable from ASTFL and LSTFL by routine histology and immunohistochemistry (IHC) alone, and has a remarkably low tendency to progress or disseminate. The cell-intrinsic alterations (e.g. mutations) and cell-extrinsic factors (microenvironment) underlying the distinctive clinical behavior of PIFL are unknown.
Patients and Methods: A total of 38 PIFL cases were available through the Kiel Lymph Node Registry and the Dana-Farber Cancer Institute (DFCI) / Massachusetts General Hospital (MGH), with clinical follow-up for 19 cases. We excluded cases of intestinal FL with evidence of mesenteric lymph node involvement or extra-intestinal manifestation. A total of 17 LSTFL cases were available from DFCI / MGH. We used 241 ASTFL cases from our previous study (Pastore, Lancet Oncology 2015) as a reference cohort.The targeted mutational landscape was determined by customized hybrid-capture targeted sequencing of 104 genes. In addition, we performed whole-exome-sequencing (WES) on 11 PIFL. The FL immune microenvironment was assessed on 8 PIFL and 7 LSTFL cases, respectively, by digital multiplexed gene expression profiling using the nCounter PanCancer Immune Profiling Panel (NanoString).
Results: PIFL patients had a median age of 57 years (range 22-84) and a male-to-female ratio of 2:3. Most cases were restricted to the duodenum (22/38, 58%). BCL2 -rearrangements and BCL2 expression by IHC was detected in 91% (20/23) and 95% (32/33) of evaluable cases, respectively. The 10-year overall survival was 100% (median follow-up of 9.1 years), and significantly superior to LSTFL and ASFL (p <0.01, respectively).
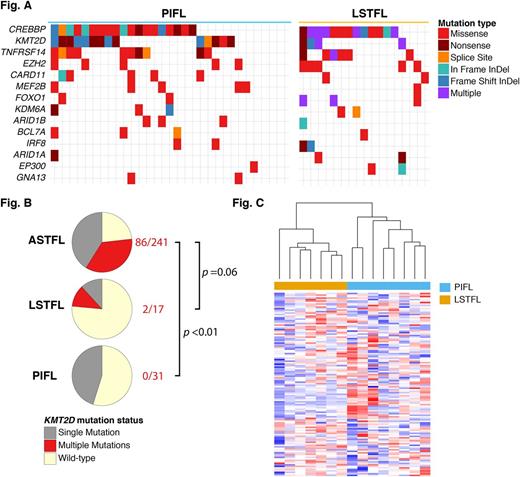
31/38 PIFL (82%) and all LSTFL (100%) cases were successfully sequenced. The mutation frequencies of the majority of recurrently mutated genes were not different between PIFL, LSTFL and ASTFL (Figure A), including CREBBP (65% vs 76% vs 70%), TNFRSF14 / HVEM (35% vs 35% vs 35%), and EZH2 (16% vs 29% vs 23%), respectively.
However, the mutation frequency of the histone lysine methyltransferase KMT2D was lower in PIFL (45%, p=0.35) and LSTFL (24%, p=0.001) compared to ASTFL (77%). Approximately one-half of KMT2D- mutated ASTFL harbored multiple, potentially biallelic mutations in this gene (86/185, 47%). In contrast, biallelic mutations were present in only 12% of LSTFL (p=0.06) and 0% of PIFL (p <0.0001; Figure B).
WES of 11 PIFL identified novel gene mutations in EEF1A1 and FLG in 2 cases, respectively. Furthermore, 4 cases harbored mutations in HVCN1 ,which have recently been associated with favorable clinical course (Krysiak, Blood 2017).
Profiling of the immune microenvironment revealed significant differences between PIFL and LSTFL. Chemokines and cytokines including CCL21, TNFSF15, CCL11, CXCL1, CXCL6 and CCL20 were strongly enriched among the top differentially expressed genes (p=0.03). Unsupervised, hierarchical clustering by expression levels of 147 chemokines and cytokines clearly separated PIFL and LSTFL into two distinct groups (Figure C).
Summary: The mutational landscape of PIFL is highly related to typical FL, both LSTFL and ASTFL. However, the lower frequency of biallelic mutations in KMT2D in PIFL and LSTFL indicates an increasing selection pressure for complete KMT2D loss during the development of, or the progression to advanced-stage FL. The highly dissimilar immune microenvironment of PIFL implies biological and clinical relevance.
Dreyling: Janssen: Consultancy, Research Funding, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Sandoz: Consultancy; Gilead: Consultancy, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; MorphoSys AG: Consultancy; Mundipharma: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal